Best Historical/Educational Cache
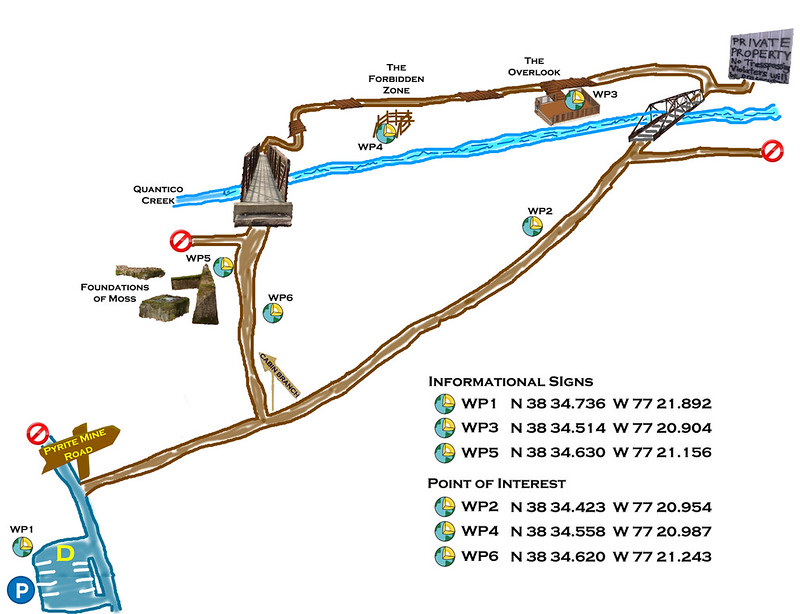
To log this cache all six listed waypoints must be visited. Cachers logging a find must send an e-mail answering the following questions;
To prove you visited the location and the waypoints associated with the practical aspects of local geology answer these three questions.
Question 1: A the first Waypoint (Listed Cache Coordinates): The sign notes that you will pass a series of two things associated with the mine. What are they?
Question 2: What do Waypoints 2, 4, and 6 have in common with the utilization of the local geology?
Question 3: At Waypoint 4 a metal post with a stamped metal disc covering its top is plainly visible from the trail. What geological site does this post mark, and who associated with VA Geology placed it?
To show some geology theory was learned answer the final three questions.
Question 4: At Waypoint 3 an informational sign notes that something was buried. What was buried? What were they buried with and why?
Question 5: At Waypoint 5 an informational sign notes the purpose of the ore. What was it?
Question 6: What elements comprise Pyrite? What geological process formed the Cabin Branch Pyrite Lens?
DO NOT PUT ANSWERS IN YOUR LOGS! Logs submitted without correct answers e-mailed to the cache owner will be deleted.
For convenience a pictorial representation of the trail and Waypoints is provided below.
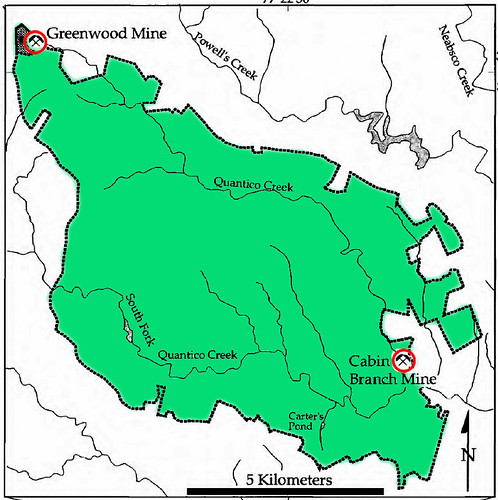
Prince William Forest Park is actually the home to two historical mines. These are the Cabin Branch Pyrite Mine and the Greenwood Gold mine. Unlike the Pyrite mine, no trail system exists around the site of the abandoned gold mine, so it can not be visited. The relationship of the two sites is seen in the picture below. The Greenwood mine was located in the area now occupied by the upper Northwest corner of Prince William Forest Park (shown in green) [1].
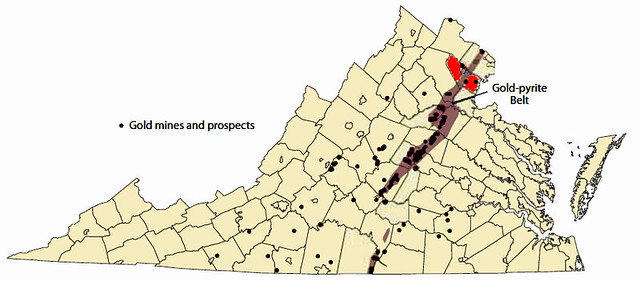
The formation of Pyrite (Fool’s Gold) and real Gold are often correlated in geology. This Earthcache will focus on Pyrite, but Gold will be mentioned on occasion due to this association. The overlap of Gold and Fool’s Gold formation is so predominant that one swath of local area is referred to as the Gold-Pyrite Belt of Virginia. This belt can be seen in the diagram below in brown, with Prince William County highlighted in red [2].
Pyrite is composed of Iron and Sulfur (whose chemical symbols are Fe and S respectively). The mineral was named for the Greek word for fire (Pyr) as it produces sparks when struck by another stone. The chemical symbol for Pyrite is FeS2 (meaning two sulfur atoms combine with every one iron atom in its formation). Today, Pyrite does not serve as an important source of either of these elements. Iron is typically obtained from oxide ores such as hematite and magnetite. These ores occur in much larger accumulations, the iron is easier to extract from them, and the metal is not contaminated with sulfur, which reduces its strength.
Pyrite used to be an important ore for the production of Sulfur and Sulfuric Acid. It can contain upwards of 53% Sulfur by weight. By the turn of the 20th century when the Cabin Branch Mine was in strong operation, Pyrite had long been one of the major sources of sulfuric acid, an important chemical agent of the time. The sulfuric acid was released from the Pyrite by heating or “roasting” the crushed ore in a chamber into which air was introduced. At high temperatures, the sulfur in the ore vaporizes and joins with the oxygen to form sulfur dioxide (SO2) gas. The sulfur dioxide was then captured and further combined with oxygen to form sulfur trioxide (SO3) which was combined with water (H2O) to manufacture sulfuric acid (H2SO4). The sulfuric acid was then used in the manufacture of glass, soap, bleach, textiles, paper, and medicine.
Of equal importance, historically pyrite served as a source for Sulfur itself, a crucial constituent of gunpowder. To recover sulfur from the Pyrite a process similar to that described above was utilized. However one crucial difference existed. The Pyrite had to be heated in the absence of air so the Sulfur would not be converted to SO2. The picture below shows Pyrite heated in a sealed pot where the sulfur vapors from the decomposing mineral could condense on the pot lid as crystals for later recovery [3].

As the US entered into World War I, Pyrite became vital for U.S. National Security. Prices per ton soared and the workers at the Cabin Branch Pyrite Mine were so needed by the war effort that they were exempted from military service.
Currently, most Sulfur is obtained as a byproduct of natural gas and crude oil processing. Some sulfur continues to be produced from Pyrite as a byproduct of Gold production. The most important use of Pyrite today is as an ore of Gold. Gold and Pyrite form under similar conditions and occur together in the same rocks. In some deposits small amounts of Gold occur as inclusions and substitutions within Pyrite. So how are both formed?
Pyrite is the most well known and widespread of the iron sulfides in surficial rocks. It occurs in rocks of every geologic age and of virtually every geologic realm, including rocks of virtually all grades of metamorphism. It is also an occasional corrosion product of ferroalloys when attacked by H2S-bearing gases or aqueous solutions. There can be little doubt that pyrite constitutes the most, or at least one of the most, dominant minerals formed or modified during a broad spectrum of ore-forming processes.
The predominance of evidence suggests that the Pyrite formed in the vicinity of the Cabin Branch Mine was the product of one dominant geologic process called “Contact Metamorphism” [4].
Contact metamorphism is the name given to the changes that take place when magma is injected into the solid rock which surrounds it (referred to as the “country rock”). This chemistry happens at the high temperatures and pressures associated with underground magma chambers coming in contact with the rocks they interface. Magma is predominantly composed of molten rock. It can also contain volatile gases and crystalline materials.
Approximately 99% of any given magma is made up of 10 elements, including Silicon (Si), Titanium (Ti), Aluminum (Al), Iron (Fe), Magnesium (Mg), Calcium (Ca), Sodium (Na), Potassium (K), Hydrogen (H) and Oxygen (O). Sulfur is one of the most abundant volatiles in terrestrial magmas. During volcanic eruptions, large amounts of sulfur are released to the atmosphere, mostly as SO2 and H2S. Sulfur also has significant effects on the partitioning of a wide variety of elements between silicate melts, liquid metals, gases, and solids, and consequently magmatic sulfur species exert major controls on the genesis of a large variety of ore deposits, such as Pyrite [5]. Depending on the location, magmas can also entrap heavier elements in their melt such as Gold.
In contact metamorphism, the changes that occur are greatest wherever the magma comes into contact with the surrounding (country) rock where the temperatures are highest, and decrease with distance from it. The reaction zones can be seen in the picture below [6].

Around the igneous rock that forms from the cooling magma is a metamorphosed zone called a contact metamorphism aureole. Aureoles may show all degrees of metamorphism from the contact area to unmetamorphosed (unchanged) country rock some distance away. The formation of ore minerals, such as Pyrite, occurs at or near the contact zone. This occurs as the constituents of the magma (such as the iron and sulfur discussed above) infiltrate into the surrounding rocks and recrystallize together.
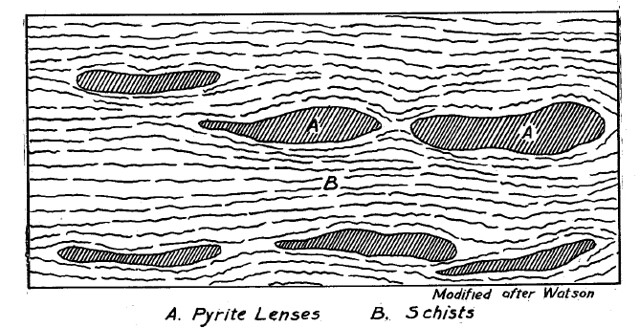
The original rock which underwent metamorphosis in the area of the Cabin Branch Mine is characterized as Schist. Schist refers to a type of metamorphic rock which is formed by the alignment of platy medium to coarse-grained minerals formed under moderate to high-grade metamorphic conditions. Schists are primarily composed of silicate minerals such as mica (muscovite and biotite), quartz, and feldspar. At the Cabin Branch Mine itself schists which were biotitic and carry quartz, hornblende, feldspar, and occasionally garnet and epidote, were the country rock (as seen in the figure below) [4].
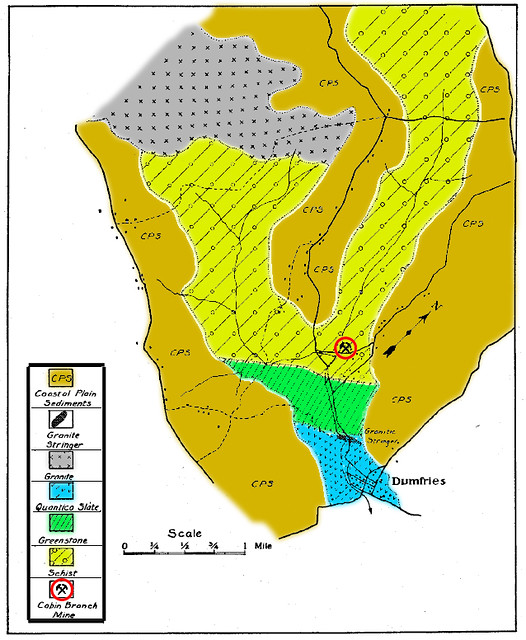
During the metamorphism, the constituents from the magma seep into the country rock and replace it with bands within its structure referred to as lenses. The essential shape of the pyrite bodies found at the Cabin Branch Mine was that of a lens. In most cases the pyrite lenses, since they are replacements, inherit their shapes from previously existing rock bodies. A cross sectional example of Pyrite Lens formation from the Gold-Pyrite Belt can be seen below [4].
The pyrite ore lens at the Cabin Branch Pyrite Mine was huge - over 1000 feet long and 1000 feet wide, with an average thickness of 14 to 18 feet.
Several factors contributed to the closing of the mine in 1920. Demand for gunpowder dropped sharply after the war. At the same time, cheaper sources of sulfur were being discovered elsewhere in the US and overseas through the new ‘global’ economy brought about by World War I. When the American Agricultural Chemical Company abandoned the Cabin Branch Pyrite Mine buildings were left vacant and the large piles of pyrite tailings (debris) were left behind. The exposed mine tailings would be left to create sulfuric acid for the next 60 years. With each rainfall, it would trickle into the Quantico Creek. By the time the National Park Service (NPS) tested the creek in 1971, the pH was 2.0 - the same as vinegar. The story of the mines remediation can be found at Way Point 3.
References;
1. Environmental Characteristics of the Abandoned Greenwood Mine Area, Prince William Forest Park, Virginia: Implications for Mercury Geochemistry, U.S. Department of the Interior U.S. Geological Survey Report 98-326, Robert R. Seal, et. al., 1998, p. 3.
2. Gold, Publication of the Virginia Department of Mines Minerals and Energy, 04/2007, Palmer Sweet, 2007, p. 1. (http://www.dmme.virginia.gov/dgmr/pdf/gold.pdf)
3. Pyrite: The Strategic Mineral That Became an Industrial Nuisance, De Re Metallica 6-7, Fathi Habashi, 2006, p. 44.
4. Geology of the Gold-Pyrite Belt of the Northeastern Piedmont Viginia, Bulletin 30 State of VA Conservation and Development Commission Virginia Geological Survey, John T. Lonsdale, 1927.
5. Sulfur in Magmas and Melts: Its Importance for Natural and Technical Processes, Reviews in Mineralogy & Geochemistry Vol. 73, 2011.
6. Metamorphic Rocks, University of Oklahoma Physical Geology. http://geophysics.ou.edu/geol1114/notes/met_rx/met_rx.html
