
From the guidelines, as from January 2013"People do not need to wait for permission to log your EarthCache. Requiring someone to wait is not supported by the EarthCache guidelines. People should send their logging task answers to you, then log your EarthCache. When you review their logging task answers, if there is a problem, you should contact them to resolve it. If there is no problem, then their log simply stands."

The word crystal is a loan from the ancient Greek word krustallos, which had the same meaning, but according to the ancient understanding of crystal, which was used to mean anything congealed by freezing, such as ice. The word once referred particularly to quartz, or "rock crystal".
Generally, in chemistry, mineralogy, and materials science, a crystal is a solid in which the constituent atoms, molecules, or ions are packed in a regularly ordered, repeating pattern extending in all three spatial dimensions.
Crystals are often characterized by their crystal system or by their crystal habit. The latter being a quicker and easier way for mineralogists and to classify what they’re observing under the microscope.
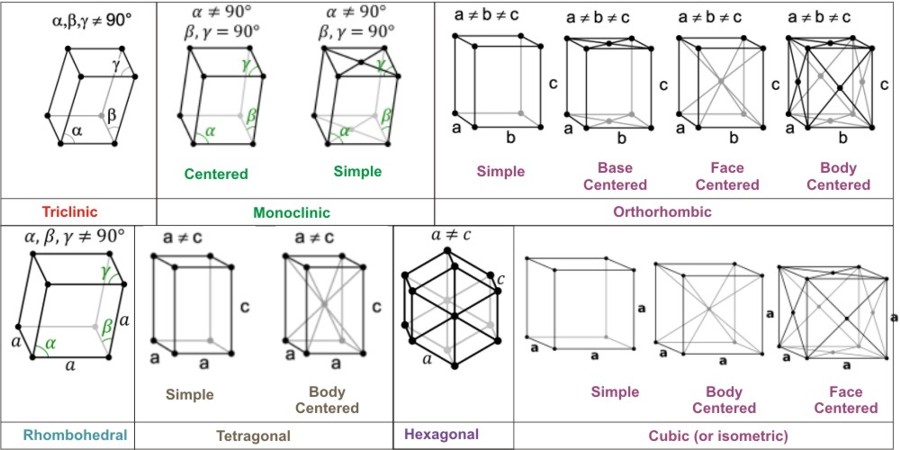
Crystal system:
A crystal system is a category of space groups, which characterise symmetry of structures in three dimensions with translational symmetry in three directions, having a discrete class of point groups.
There are 7 crystal systems:
- Triclinic, all cases not satisfying the requirements of any other system. There is no necessary symmetry other than translational symmetry, although inversion is possible.
The triclinic lattice is the least symmetric of the 14 three-dimensional Bravais lattices. It has (itself) the minimum symmetry all lattices have: points of inversion at each lattice point and at 7 more points for each lattice point: at the midpoints of the edges and the faces, and at the center points. It is the only lattice type that itself has no mirror planes.
- Monoclinic, requires either 1 twofold axis of rotation or 1 mirror plane.
In the monoclinic system, the crystal is described by vectors of unequal length, as in the orthorhombic system. They form a rectangular prism with a parallelogram as its base. Hence two pairs of vectors are perpendicular, while the third pair make an angle other than 90°.
- Orthorhombic, requires either 3 two-fold axes of rotation or 1 two-fold axis of rotation and two mirror planes.
Orthorhombic lattices result from stretching a cubic lattice along two of its lattice vectors by two different factors, resulting in a rectangular prism with a rectangular base (a by b) and height (c), such that a, b, and c are distinct. All three bases intersect at 90° angles. The three lattice vectors remain mutually orthogonal.
- Tetragonal, requires 1 fourfold axis of rotation.
Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (a by a) and height (c, which is different from a).
- Rhombohedral, also called trigonal, requires 1 threefold axis of rotation.
In the rhombohedral system, the crystal is described by vectors of equal length, of which all three are not mutually orthogonal. The rhombohedral system can be thought of as the cubic system stretched diagonal along a body. a = b = c; alpha=beta=theta not equal to 90º . In some classification schemes, the rhombohedral system is grouped into a larger hexagonal system.
- Hexagonal, requires 1 sixfold axis of rotation.
It has the same symmetry as a right prism with a hexagonal base. There is only one hexagonal Bravais lattice, which has six atoms per unit cell.
- Isometric or cubic, requires 4 threefold axes of rotation.
The cubic crystal system (or isometric) is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
Crystal Habit:
In mineralogy, shape and size give rise to descriptive terms applied to the typical appearance, or habit of crystals.
The many terms used by mineralogists to describe crystal habits are useful in communicating what specimens of a particular mineral often look like. Recognizing numerous habits helps a mineralogist to identify a large number of minerals. Some habits are distinctive of certain minerals, although most minerals exhibit many differing habits (the development of a particular habit is determined by the details of the conditions during the mineral formation/crystal growth). Crystal habit may mislead the inexperienced as a mineral's internal crystal system can be hidden or disguised.
Factors influencing a crystal's habit include: a combination of two or more crystal forms; trace impurities present during growth; crystal twinning and growth conditions (i.e., heat, pressure, space). Minerals belonging to the same crystal system do not necessarily exhibit the same habit. Some habits of a mineral are unique to its variety and locality: For example, while most sapphires form elongate barrel-shaped crystals, those found in Montana form stout tabular crystals. Ordinarily, the latter habit is seen only in ruby (Sapphire and ruby are both varieties of the same mineral; corundum).
Some minerals may replace other existing minerals while preserving the original's habit: this process is called pseudomorphous replacement. A classic example is tiger's eye quartz, crocidolite asbestos replaced by silica. While quartz typically forms euhedral (well-formed), prismatic (elongate, prism-like) crystals, in tiger's eye the original fibrous habit of crocidolite is preserved.
|
Habit
|
Description
|
Example
|
|
Acicular
|
Needle-like, slender and/or tapered
|
Rutile in quartz
|
|
Amygdaloidal
|
Almond-shaped
|
Heulandite
|
|
Anhedral
|
Poorly formed, external crystal faces not developed
|
Olivine
|
|
Bladed
|
Blade-like, slender and flattened
|
Kyanite
|
|
Botryoidal or globular
|
Grape-like, hemispherical masses
|
Smithsonite,Hemimorphite,Adamite and Variscite
|
|
Columnar
|
Similar to fibrous: Long, slender prisms often with parallel growth
|
Calcite
|
|
Coxcomb
|
Aggregated flaky or tabular crystals closely spaced
|
Barite
|
|
Dendritic or arborescent
|
Tree-like, branching in one or more direction from central point
|
Magnesite in opal
|
|
Dodecahedral
|
Dodecahedron, 12-sided
|
Garnet
|
|
Drusy or encrustation
|
Aggregate of minute crystals coating a surface
|
Uvarovite
|
|
Enantiomorphic
|
Mirror-image habit and optical characteristics; right- and left-handed crystals
|
Quartz
|
|
Equant, stout, stubby or blocky
|
Length, width, and breadth roughly equal
|
Zircon
|
|
Euhedral
|
Well-formed, external crystal faces developed
|
Spinel
|
|
Fibrous or columnar
|
Extremely slender prisms
|
Tremolite
|
|
Filiform or capillary
|
Hair-like or thread-like, extremely fine
|
Natrolite
|
|
Foliated or micaceous
|
Layered structure, parting into thin sheets
|
Mica
|
|
Granular
|
Aggregates of anhedral crystals in matrix
|
Scheelite
|
|
Hemimorphic
|
Doubly terminated crystal with two differently shaped ends
|
Hemimorphite
|
|
Mamillary
|
Breast-like: surface formed by intersecting partial spherical shapes
|
Malachite
|
|
Massive or compact
|
Shapeless, no distinctive external crystal shape
|
Serpentine
|
|
Nodular or tuberose
|
Deposit of roughly spherical form with irregular protuberances
|
Geodes
|
|
Octahedral
|
Octahedron, eight-sided (two pyramids base to base)
|
Diamond
|
|
Plumose
|
Fine, feather-like scales
|
Mottramite
|
|
Prismatic
|
Elongate, prism-like: crystal faces parallel to c-axis
|
well-developed Tourmaline
|
|
Pseudo-hexagonal
|
hexagonal appearance due to cyclic twinning
|
Aragonite
|
|
Pseudomorphous
|
Occurring in the shape of another mineral through pseudomorphous replacement
|
Tiger's eye
|
|
Radiating or divergent
|
Radiating outward from a central point
|
Pyrite suns
|
|
Reniform or colloform
|
Similar to mamillary: intersecting kidney-shaped masses
|
Hematite
|
|
Reticulated
|
Acicular crystals forming net-like intergrowths
|
Cerussite
|
|
Rosette
|
Platy, radiating rose-like aggregate
|
Gypsum
|
|
Sphenoid
|
Wedge-shaped
|
Sphene
|
|
Stalactitic
|
Forming as stalactites or stalagmites; cylindrical or cone-shaped
|
Rhodochrosite
|
|
Stellate
|
Star-like, radiating
|
Pyrophyllite
|
|
Striated/striations
|
Surface growth lines parallel or perpendicular to a crystallographic axis
|
Chrysoberyl
|
|
Subhedral
|
External crystal faces only partially developed Tabular or lamellar Flat, tablet-shaped, prominent pinnacoid
|
Ruby
|
|
Wheat sheaf
|
Aggregates resembling hand-reaped wheat sheaves
|
Zeolites
|
Ore minerals and Crystals
Ore is defined as a volume of rock containing components or minerals in a mode of occurrence that renders it valuable for mining. An ore must contain materials that are 1- valuable and 2- present in concentrations that can be profitably mined, transported, milled, and processed.
Ore minerals are generally oxides, sulphides, silicates, or "native" metals (such as native copper) that are not commonly concentrated in the Earth's crust or "noble" metals (not usually forming compounds) such as gold.
Metals occur, at times, in perfect crystal forms. Everyone has already seen pyrite cubes in minerals shops (as in the image) but many other ore minerals occur in crystal form if formed under the right conditions (see image).

The cache
The above coordinates will take you to a traffic circle in Castro Verde in the Alentejo. This traffic circle is filled with crystals (very large ones!).
Here you will need to identify
1- How many different types of minerals are exhibited here,
2- What colour are they?,
3- Given the setting where these statues have been placed, identify what metals are represented at this point and finally,
4- Tell what crystal systems do you think these crystals crystallise in.
E-mail me the answers through my GC profile to validate your found.
NO tell-tale photos in the logs.
Have fun!

A cache:
As coordenadas desta cache levam-te a uma rotunda no meio de Castro Verde no Alentejo. Esta rotunda está pejada de cristais bem grandes onde para conseguires logar a cache tens de:
1- Identificar quantos tipos de cristais estão expostos no local,
2- Qual a côr dos cristais?,
3- Quais os metais(*) que estes cristais representam? e,
4- A que sistema cristalográfico pertencem?
(*) Para conseguirem responder a esta pergunta, pensem um pouco do local onde está a estátua e porquê?
Enviem-me uma mensagem com as devidas respostas para validar o vosso found.
Divirtam-se!
 The most exciting way to learn about the Earth and its processes is to get into the outdoors and experience it first-hand. Visiting an Earthcache is a great outdoor activity the whole family can enjoy. An Earthcache is a special place that people can visit to learn about a unique geoscience feature or aspect of our Earth. Earthcaches include a set of educational notes and the details about where to find the location (latitude and longitude). Visitors to Earthcaches can see how our planet has been shaped by geological processes, how we manage the resources and how scientists gather evidence to learn about the Earth. To find out more click HERE.
The most exciting way to learn about the Earth and its processes is to get into the outdoors and experience it first-hand. Visiting an Earthcache is a great outdoor activity the whole family can enjoy. An Earthcache is a special place that people can visit to learn about a unique geoscience feature or aspect of our Earth. Earthcaches include a set of educational notes and the details about where to find the location (latitude and longitude). Visitors to Earthcaches can see how our planet has been shaped by geological processes, how we manage the resources and how scientists gather evidence to learn about the Earth. To find out more click HERE.
______________________________________________________________________________
